In scientific studies, particularly those related to vaccines, pharmaceuticals, or public health interventions, certain phrases and statistical tricks are used to obfuscate findings, exaggerate benefits, or downplay risks.
Below are a few of the most common red flag phrases to look out for, along with examples from vaccine studies where positive-sounding conclusions did not match the actual data.
- Red Flag Phrases That Overstate Benefits
🚩 “The Vaccine is 95% Effective” (Relative Risk vs. Absolute Risk Misrepresentation)
Example: Pfizer COVID-19 Vaccine Trial (NEJM, 2020)
• The media reported that the vaccine was 95% effective, but this was relative risk reduction (RRR), not absolute risk reduction (ARR).
• The absolute risk reduction (ARR) was only about 0.7%, meaning out of 100 people, less than 1 person actually benefited.
• Key Red Flag: If a study reports relative risk reduction (RRR) but hides absolute risk reduction (ARR), it is inflating the perceived benefit.
🚩 “The Vaccine Prevented X% of Cases” (Without Stating the Baseline Risk)
Example: HPV Vaccine (Gardasil) Trials
• The study claimed the vaccine prevented 100% of HPV-related pre-cancerous lesions.
• However, the baseline risk for these lesions was already incredibly low (0.1% risk in the general population).
• If the baseline risk is near zero, even a high “risk reduction” is meaningless.
Key Red Flag: Always ask, “What was the baseline risk?” A high reduction in a nearly nonexistent risk is not real-world evidence of benefit.
- Red Flag Phrases That Hide Harm or Negative Findings
🚩“The Vaccine Was Well-Tolerated” (Without Defining What “Well-Tolerated” Means)
Example: Moderna COVID-19 Vaccine Trial (NEJM, 2020)
• The study stated the vaccine was “well-tolerated with mild-to-moderate side effects.”
Actual data showed that:
• 82% of participants reported moderate-to-severe reactions (fatigue, headaches, muscle pain).
• Severe adverse events (Grade 3/4) occurred in 15% of recipients.
Key Red Flag: If a study claims an intervention is “well-tolerated,” but the actual adverse event data is high, this is misleading language.
🚩“There Were No Serious Adverse Events Related to the Vaccine”
Example: Gardasil HPV Vaccine Trials (FDA Review, 2006)
• Many participants developed autoimmune diseases post-vaccination.
• However, researchers labeled these events as “not related to the vaccine.”
Key Red Flag: If a study states “no serious adverse events related to the vaccine,” check who determined that the events were unrelated. Often, the data shows significant harm that is dismissed due to arbitrary classifications.
- Red Flag Phrases That Manipulate Data Analysis
🚩“The Data Suggests a Trend Toward Efficacy” (When Results Are Statistically Insignificant)
Example: Influenza Vaccine Trials in the Elderly
• Many flu vaccine studies conclude that “there is a trend toward lower hospitalization rates.”
Key Red Flag: If a study uses the phrase “trend toward efficacy,” it means the results were not statistically significant, and they are stretching the interpretation to suggest benefit.
🚩“The Vaccine May Provide Protection Against Severe Disease” (Speculative Claim Without Hard Data)
Example: COVID-19 Vaccine Trials Post-Approval
• Many post-market studies claimed the vaccines “reduce severe disease” despite lack of hard clinical evidence.
• Hospitalization data was often misclassified, as mild COVID cases in vaccinated individuals were often recorded as “moderate/severe” due to hospitalization for monitoring.
Key Red Flag: If a study says a treatment “may provide protection,” check if the data actually supports the claim, or if they are making speculative conclusions.
- Red Flag Phrases That Obscure Study Design Issues
🚩“More Research is Needed to Confirm These Findings” (Despite Strong Data Already Showing a Risk or Harm)
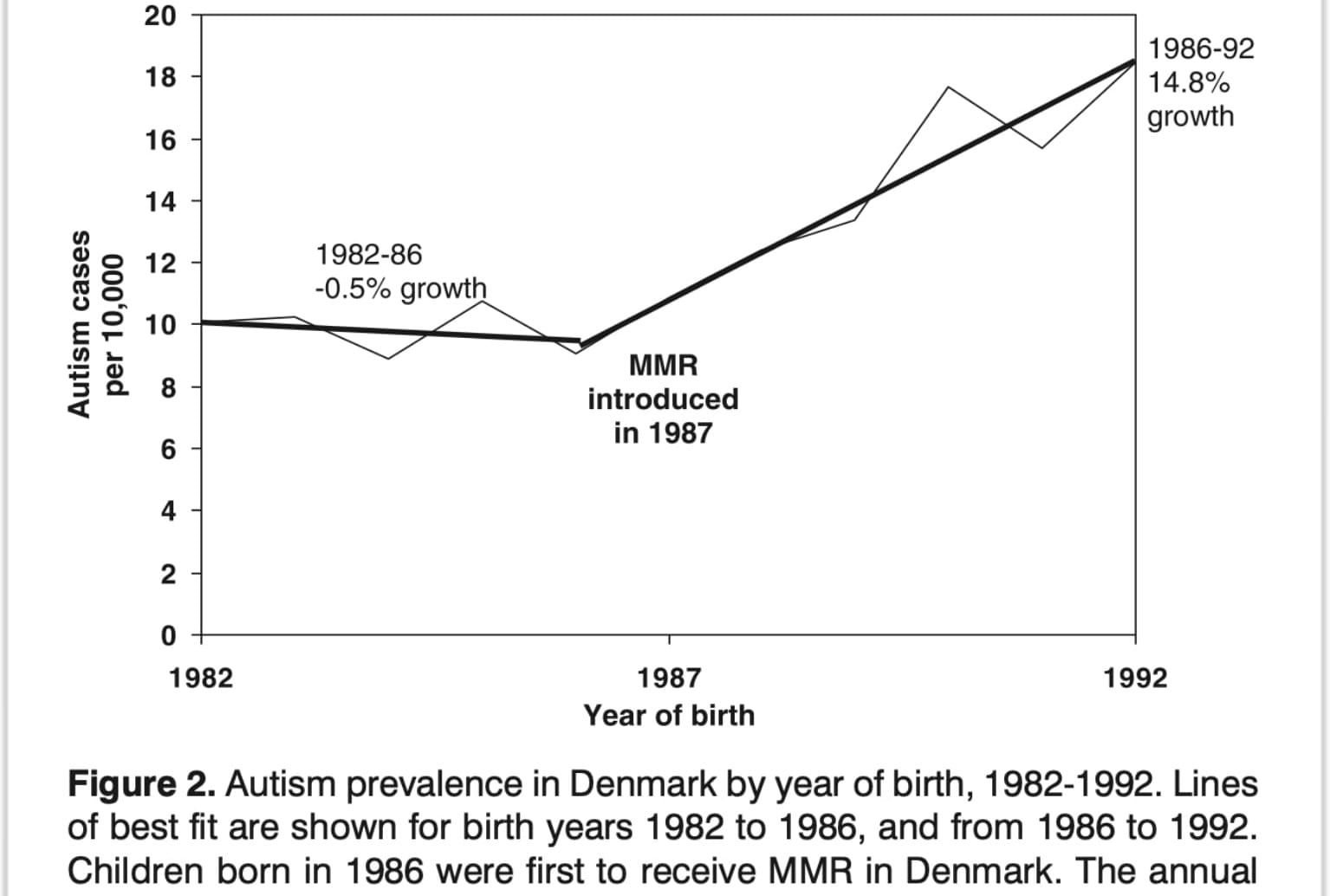
Example: COVID-19 Myocarditis Studies in Young Males
• Early studies showed a clear link between mRNA vaccines and myocarditis in young males.
• However, many official reports concluded, “more research is needed” rather than admitting the risk.
Key Red Flag: If strong evidence already exists but a study avoids drawing firm conclusions, this is often a tactic to delay action on findings that contradict industry narratives.
🚩 “The Vaccine Was Tested in a Diverse Population” (When the Cohort Was Selectively Chosen)
Example: COVID-19 Vaccine Trials Excluding Pregnant Women & Children
• Despite not testing the vaccine in pregnant women or children, regulatory agencies still approved its use in these groups.
Key Red Flag: If a study claims diversity but excludes key risk groups, the results do not apply to the real-world population.
- Red Flag Phrases That Bury Negative Results in the Study’s Structure
🚩 “Participants Who Did Not Complete the Study Were Excluded From Analysis”
Example: Pfizer COVID-19 Trial (NEJM, 2020)
• The study excluded 3,410 participants who “did not meet protocol criteria.”
Key Red Flag: If a study excludes large numbers of participants, especially those with negative outcomes, it is likely manipulating the data to inflate efficacy and downplay harm.
🚩“Vaccine Effectiveness Was Calculated Based on PCR-Confirmed Cases” (When PCR Test Sensitivity is an Issue)
Example: COVID-19 Vaccine Trials Using High-Cycle PCR Tests
• Many COVID-19 trials classified infections based on high-cycle PCR testing, which detects non-infectious viral fragments, inflating case numbers.
Key Red Flag: If a study relies heavily on PCR-confirmed cases without specifying cycle thresholds (CT values), the data may be unreliable.
How to Read Studies Critically: Key Questions to Ask
- How is risk reduction being reported? (Absolute risk vs. relative risk)
- Are there any excluded participants or missing data?
- Are the conclusions based on speculation rather than hard statistical findings?
- Was the study funded by an industry with a financial interest in the results?
- Were long-term effects actually measured, or was the study too short to detect them?
- Did they adjust for confounding variables (e.g., healthy user bias in vaccine studies)?
Science Should Be Transparent, Not Manipulative
- If a study avoids making direct, data-driven conclusions and instead uses vague, subjective language, that is a major red flag.
- If a study presents impressive percentage reductions without absolute numbers, it is inflating results.
- If a study claims “no significant evidence” of harm but the raw data suggests otherwise, it is likely downplaying risks.
Scientific integrity requires clear data, transparent methodology, and unbiased conclusions. Whenever we see these red flag phrases, we should dig deeper—because more often than not, the truth is buried beneath the verbiage.






Discussion