
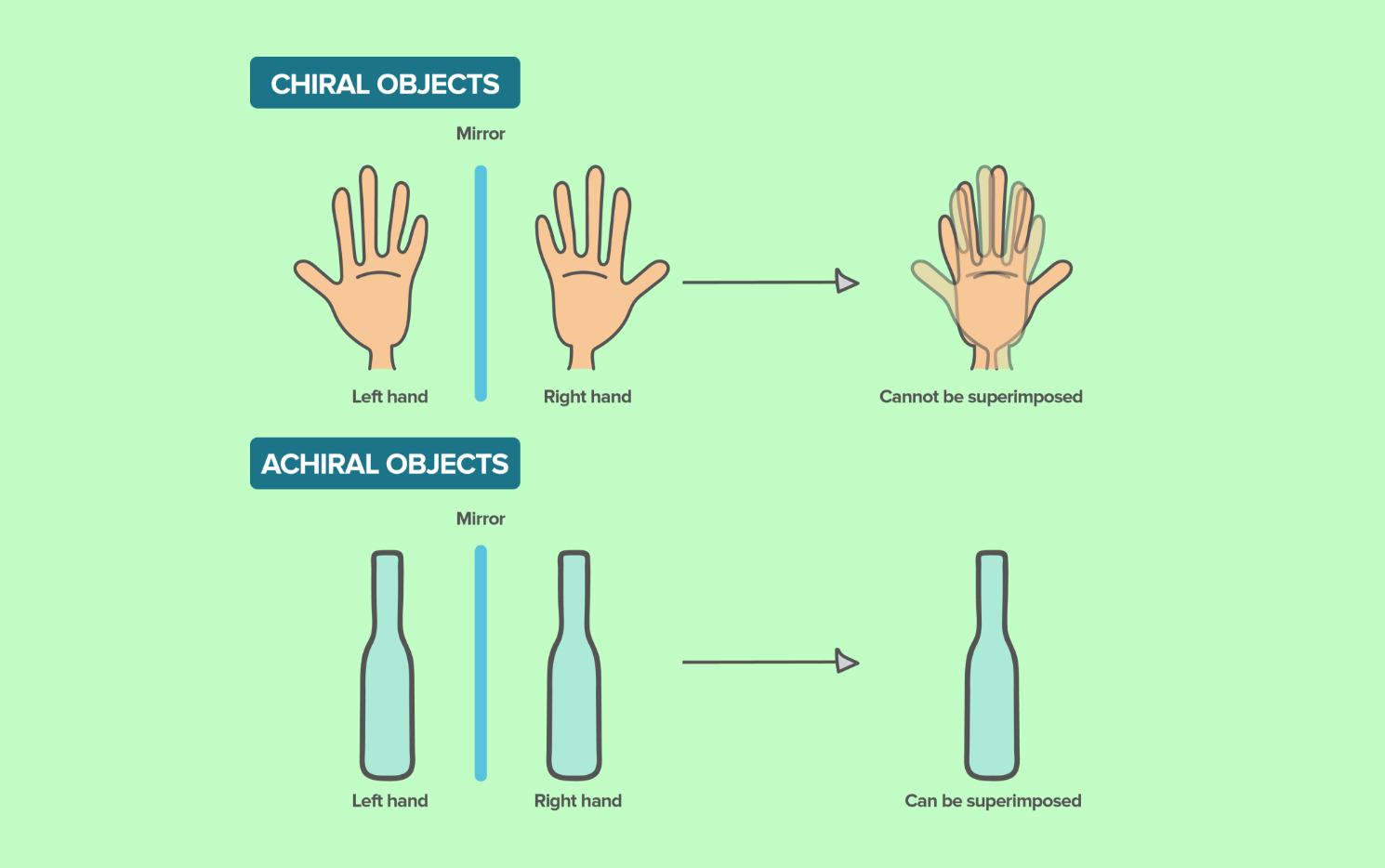
Everything alive on Earth is chiral.
Chirality means that a molecule and its mirror image are not superimposable, like your left and right hands. This “handedness” gives biological molecules specific orientations that deeply matter for how life functions.
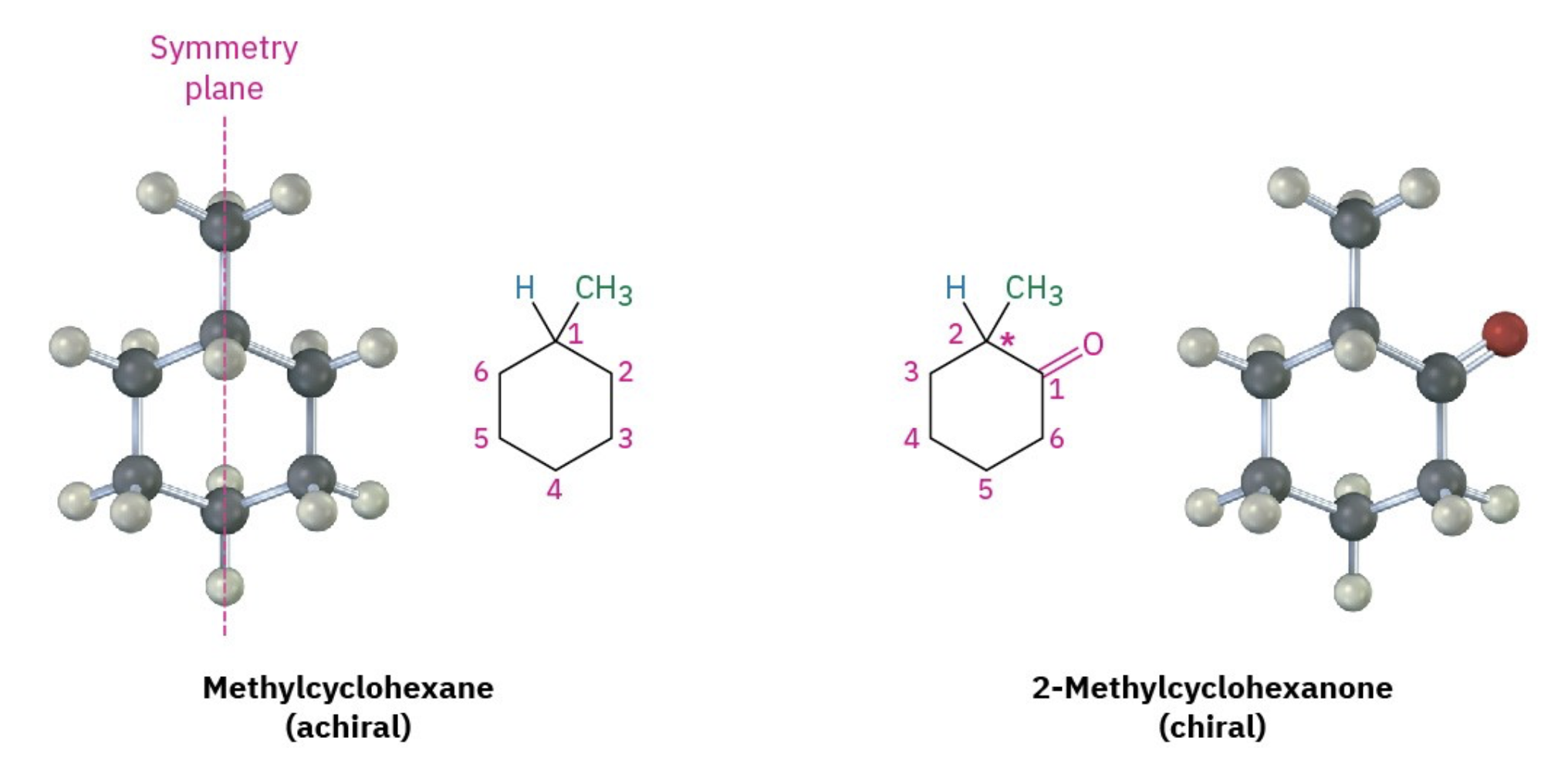
All known biological life uses exclusively left-handed amino acids (L-enantiomers) and right-handed sugars (D-enantiomers). These asymmetrical structures allow for selective binding, precise enzyme function, cellular signaling, and molecular recognition. In biological systems, these chiral interactions are essential for coherence and function.
Life is made of asymmetries that cooperate to generate dynamic symmetry, not a static, frozen balance, but a living, responsive coherence across scales. This coherence depends on directionality, pattern, and orientation.
Molecules that lack chirality (achiral substances) may not meaningfully participate in these selective biological interactions. While some achiral molecules, like water and oxygen, are vital for life, their roles tend to be structurally neutral or mediating, rather than directly encoded with directional biochemical information.
In contrast, synthetic compounds that do not replicate the specific chirality of natural molecules may fail to engage appropriately with biological systems.
Though they may mimic structural form, their interaction with the body's energetic or field-based organization may differ significantly. This is an area of growing investigation, especially in the context of pharmacology, where different enantiomers of a drug can produce very different effects.
Cells themselves are not symmetrical spheres. They are polarized and chiral in 3D. They exhibit structured polarity along three axes: apical-basal (top-bottom), front-rear, and left-right.
Each axis of polarity breaks symmetry in a specific direction, enabling key processes like absorption, secretion, locomotion, orientation, and differentiation. This polarity is not random, it’s encoded and maintained energetically, biochemically, and structurally.
- Apical-basal polarity lets cells distinguish between their outer and inner environments, breaking vertical symmetry.
- Front-rear polarity gives direction to movement and orientation, breaking anterior-posterior symmetry.
- Left-right polarity is essential in development, such as how organs know where to form, breaking bilateral symmetry.
So, from the level of the molecule to the cell to the whole organism, chirality and polarity scale upward. They create systems that are asymmetric in parts but coordinated in function.
Zooming further out, this same principle plays out at macroscopic levels. Organs like the heart or liver are asymmetrically placed, yet they serve systemic roles that maintain overall energetic symmetry.
The human body appears bilaterally symmetrical externally, but is internally structured with layered asymmetries that enable balance and coherence. Planet Earth follows this pattern too: from afar it seems symmetrical, a sphere with magnetic poles and orbital regularity, but internally it is full of dynamic imbalances: currents, crusts, climates, and fields, all organized into coherence.
In short, coherence in biology is not sameness; it’s resonance across structured difference. It is this tuning of asymmetries into alignment that generates life.
This concept finds deep support in physics, too. Noether’s theorem shows that for every symmetry in a system, there is a conserved quantity. Translational symmetry conserves momentum; time symmetry conserves energy. But these conserved quantities only persist when the system’s components interact through underlying fields and structure.
Likewise in biology, the directional interactions made possible by chirality help preserve coherence through change. When chirality is disrupted, those fields of coordination may be impaired.
Every “alive” process in the body is chiral-dependent, because chirality enables directionality, which enables field coupling.
Here is the most important thing to understand about chirality: man cannot produce anything - molecules, DNA, vaccines, vitamins, hormones - our scientists are only capable of replicating the form or structure of these, they have never been able to replicate the chiral properties.
We are incapable of engineering chiral molecules with quantum properties that align with the quantum properties of the natural substance. We know how to copy the form, but we have no way of replicating the function or frequency.
The problem with racemates is how they interact with light and energy. A molecule with chirality rotates plane-polarized light, but a racemate (the equal mix of both achiral and chiral), doesn't rotate light.

All synthetic materials can look identical under a microscope, but they act completely different because they are disconnected from the field-based intelligence of life. Synthetic pharmacology assumes cells are symmetrical blobs. But real cells are chiral, polarized, and field-sensitive. This is why even “identical” molecules can behave oppositely based on entry point, electric charge, or biofield orientation.
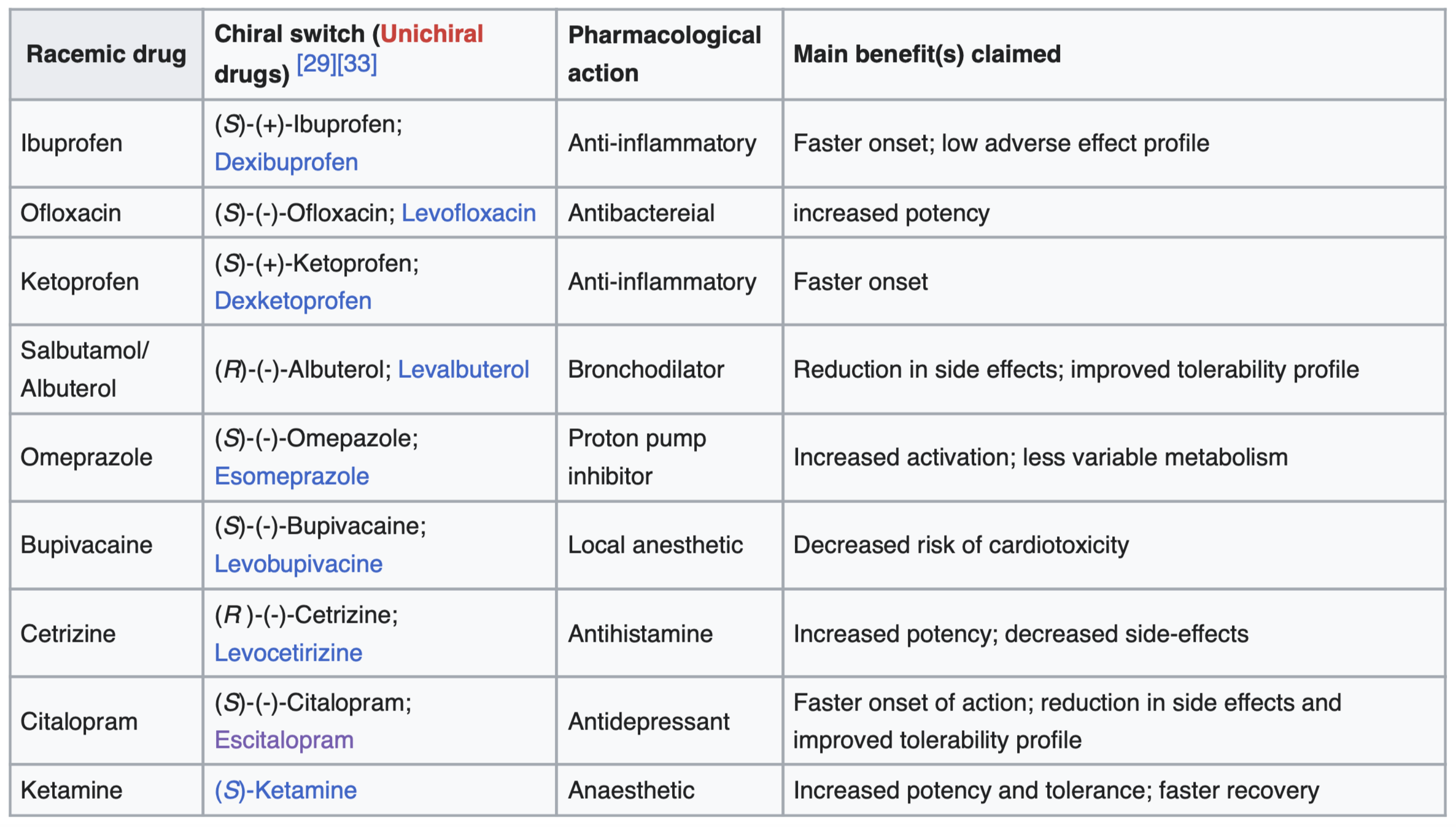
For example, the case of thalidomide demonstrated how two enantiomers of the same drug had radically different biological effects, one beneficial, the other harmful. It reminded science that symmetry and asymmetry at the molecular level can be the difference between healing and harm.
It is also why pharmaceutical design increasingly seeks to isolate or synthesize the biologically active enantiomer rather than a racemic mixture (equal mix of both left- and right-handed forms). Even when molecules look identical under a microscope, their behavior can differ depending on their orientation, their energy interactions, and their compatibility with biological systems.










Discussion